Ethylene oxide
| Ethylene oxide | |
|---|---|
 |
 |
|
oxirane [1]
|
|
|
Other names
epoxyethane, ethylene oxide, dimethylene oxide,oxacyclopropane
|
|
| Identifiers | |
| Abbreviations | EO, EtO |
| CAS number | 75-21-8 |
| PubChem | 6354 |
| EC number | 200-849-9 |
| KEGG | C06548 |
| MeSH | Ethylene+Oxide |
| ChEBI | 27561 |
| RTECS number | KX2450000 |
|
SMILES
C1CO1
|
|
|
InChI
InChI=1/C2H4O/c1-2-3-1/h1-2H2
|
|
| Properties | |
| Molecular formula | C2H4O |
| Molar mass | 44.05 g mol−1 |
| Appearance | colorless gas |
| Density | 0.882 g/mL, 7.360 lbs/gallon |
| Melting point |
−111.3 °C |
| Boiling point |
10.7 °C |
| Solubility in water | miscible |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−52.6 kJ mol−1 |
| Standard molar entropy S |
243 J mol−1 K−1 |
| Hazards | |
| Main hazards | carcinogen |
| NFPA 704 |
 4
3
3
|
| Flash point | −20 °C |
| Explosive limits | 3 to 100% |
| Related compounds | |
| Related heterocycles | Aziridine, Ethylene sulfide, Borirane |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Ethylene oxide, also called oxirane, is the organic compound with the formula C2H4O. This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered ring consisting of two carbon and one oxygen atom. Because of its special molecular structure, ethylene oxide easily participates in the addition reaction, opening its cycle, and thus easily polymerizes. Ethylene oxide is isomeric with acetaldehyde. It is a strong poison to humans, showing carcinogenic, mutagenic, irritating and narcotic effects.
The major application of ethylene oxide is for producing many chemicals and intermediates, such as ethylene glycol, ethanolamines, simple and complex glycols, polyglycol ethers and other compounds. It is also a common gas-phase disinfectant which is widely used in hospitals to sterilize heat-sensitive tools and equipment.[1] Ethylene oxide is industrially produced by direct oxidation of ethylene in the presence of silver catalyst. It is extremely flammable and explosive and is used as a main component of thermobaric weapons;[2][3] therefore, it is commonly handled and shipped as a refrigerated liquid.[4]
History
Ethylene oxide was first reported in 1859 by the French chemist Charles-Adolphe Wurtz,[5] who prepared it by treating 2-chloroethanol with potassium hydroxide:
- Cl–CH2CH2–OH + KOH → (CH2CH2)O + KCl + H2O
Wurtz measured the boiling point of ethylene oxide as 13.5 °C, slightly higher than the present value, and discovered the ability of ethylene oxide to react with acids and salts of metals.[6] Wurtz mistakenly assumed that ethylene oxide has the properties of an organic base. This delusion lasted until 1896 when Georg Bredig found that ethylene oxide is not an electrolyte.[6] Its distinct difference with ethers, in particular, its propensity to join the addition reactions typical of unsaturated compounds, had long been a matter of debate. Only in 1893, the heterocyclic triangular structure of ethylene oxide had been proposed.[6]
The first synthesis method had long remained the only, despite numerous attempts of scientists, including Wurtz himself, to produce ethylene oxide directly from ethyl.[7] Only in 1931, French chemist Theodore Lefort developed a method of direct oxidation of ethylene in the presence of silver catalyst.[8] Since 1940, almost all ethylene oxide produced industrially uses this method.[9] Sterilization by ethylene oxide for the preservation of spices was patented in 1938 by the American chemist Lloyd Hall. Ethylene oxide achieved industrial importance during World War I as a precursor to both the coolant ethylene glycol and the chemical weapon mustard gas.
Molecular structure and properties

The epoxy cycle of ethylene oxide is an almost regular triangle with bond angles of about 60° and a significant angular stress corresponding to the energy of 105 kJ/mol.[10][11] For comparison, in alcohols the C–O–H angle is about 110°; in ethers, the C–O–C angle is 120°. The moment of inertia about the principal axes are IA = 32.921×10−40 g·cm², IB = 37.926×10−40 g·cm² and IC = 59.510×10−40 g·cm².[12] The dipole moment at a temperature in the range 17–176 °C is 6.26×10−30 C·m.[13]
The relative instability of the carbon-oxygen bonds in the molecule is revealed by the comparison in the table of the energy required to break two C–O bonds in the ethylene oxide or one C–O bond in ethanol and dimethyl ether:[14]
| Reaction | ΔH°298, kJ/mol | Method |
|---|---|---|
| (C2H4)O → C2H4 + O (cleavage of two bonds) | 354.38 | Calculated, from atomic enthalpies |
| C2H5OH → C2H5 + OH (breaking one bond) | 405.85 | Electron impact |
| CH3OCH3 → CH3O + CH3 (breaking one bond) | 334.72 | Calculated using enthalpies of radicals formation |
This instability determines the chemical activity of ethylene oxide and explains the ease of opening its cycle in addition reactions (see Chemical properties).
Physical properties
Ethylene oxide is a colorless gas at 25 °C and is a mobile liquid at 0 °C – viscosity of liquid ethylene oxide at 0 °C is about 5.5 times lower than that of water. The gas has a characteristic sweet odor of ether, noticeable when its concentration in air exceeds 500 ppm.[15] Ethylene oxide is readily soluble in water, ethanol, diethyl ether and many organic solvents.[16]
Main thermodynamical constants are:[17]
- Standard molar heat capacity, Cp° = 48.19 J/(mol·K);
- Standard enthalpy of formation, ΔH°298 = –51.037 kJ/mol;
- Standard entropy, S°298 = 243.4 J/(mol·K);
- Gibbs free energy, ΔG°298 = –11.68 kJ/mol;
- Heat of combustion, ΔHc° = –1306 kJ/mol.[18]
The surface tension of liquid ethylene oxide, at the interface with its own steam, is 35.8 mJ/m2 at –50.1 °C and 27.6 mJ/m2 at –0.1 °C.[19]
The boiling point increases with the vapor pressure as follows:[20] 57.7 (2 atm), 83.6 (5 atm) and 114.0 (10 atm).
Viscosity decreases with temperature with the values of 0.577 kPa·s at –49.8 °C, 0.488 kPa·s at –38.2 °C, 0.394 kPa·s at –21.0 °C and 0.320 kPa·s at 0 °C.[21]
Between –91 °C and 10.5 °C, vapor pressure p (in mmHg) varies with temperature (T in °C) as lg p = 6.251 – 1115.1/(244.14 + T).[22]
| Temperature, °C | Steam pressure, kPa | Enthalpy of the liquid, J/g |
Enthalpy of vaporization, J/g |
Density, kg/L | Heat capacity, J/(kg·K) | Thermal conductivity, W/(m·K) |
|---|---|---|---|---|---|---|
| –40 °C | 8.35 | 0 | 628.6 | 0.9488 | 1878 | 0.20 |
| –20 °C | 25.73 | 38.8 | 605.4 | 0.9232 | 1912 | 0.18 |
| 0 °C | 65.82 | 77.3 | 581.7 | 0.8969 | 1954 | 0.16 |
| 20 °C | 145.8 | 115.3 | 557.3 | 0.8697 | 2008 | 0.15 |
| 40 °C | 288.4 | 153.2 | 532.1 | 0.8413 | 2092 | 0.14 |
| 60 °C | 521.2 | 191.8 | 505.7 | 0.8108 | 2247 | 0.14 |
| 80 °C | 875.4 | 232.6 | 477.4 | 0.7794 | 2426 | 0.14 |
| 100 °C | 1385.4 | 277.8 | 445.5 | 0.7443 | 2782 | 0.13 |
| 120 °C | 2088 | 330.4 | 407.5 | 0.7052 | 3293 | N/A* |
| 140 °C | 3020 | 393.5 | 359.4 | 0.6609 | 4225 | N/A |
| 160 °C | 4224 | 469.2 | 297.1 | 0.608 | N/A | N/A |
| 180 °C | 5741 | 551.2 | 222.5 | 0.533 | N/A | N/A |
| 195.8 °C | 7191 | N/A | N/A | N/A | N/A | N/A |
*N/A – data not available.
| Temperature, K | Entropy, J/(mol·K) | Heat of formation, kJ/mol | Free energy of formation, kJ/mol | Viscosity Pa·s | Thermal conductivity, W/(m·K) | Heat capacity, J/(mol·K) |
|---|---|---|---|---|---|---|
| 298 | 242.4 | –52.63 | –13.10 | N/A | N/A | 48.28 |
| 300 | 242.8 | –52.72 | –12.84 | 9.0 | 0.012 | 48.53 |
| 400 | 258.7 | –56.53 | 1.05 | 13.5 | 0.025 | 61.71 |
| 500 | 274.0 | –59.62 | 15.82 | 15.4 | 0.038 | 75.44 |
| 600 | 288.8 | –62.13 | 31.13 | 18.2 | 0.056 | 86.27 |
| 700 | 302.8 | –64.10 | 46.86 | 20.9 | 0.075 | 95.31 |
| 800 | 316.0 | –65.61 | 62.80 | N/A | 0.090 | 102.9 |
*N/A – data not available.
Chemical properties
Ethylene oxide readily reacts with various compounds, breaking a C–O bond and opening the cycle. Its typical reactions are with nucleophiles which proceed via the SN2 mechanism both in acidic (weak nucleophiles: water, alcohols) and alkaline media (strong nucleophiles: OH–, RO–, NH3, RNH2, RR'NH, etc.).[11] The general reaction scheme is
and more specific reactions are described below.
Addition of water and alcohols
Aqueous solutions of ethylene oxide are rather stable and can exist for a long time without any noticeable chemical reaction, but adding a small amount of acid, such as strongly diluted sulfuric acid, immediately leads to the formation of ethylene glycol, even at room temperature:
- (CH2CH2)O + H2O → HO–CH2CH2–OH
The reaction also occurs in the gas phase, in the presence of a phosphoric acid salt as a catalyst.[23]
The reaction is usually carried out at about 60 °C with a large excess of water, in order to prevent the reaction of the formed ethylene glycol with ethylene oxide that would form di- and triethylene glycol:[24]
- 2 (CH2CH2)O + H2O → HO–CH2CH2–O–CH2CH2–OH
- 3 (CH2CH2)O + H2O → HO–CH2CH2–O–CH2CH2–O–CH2CH2–OH
The use of alkaline catalysts may lead to the formation of polyethylene glycol:
- n (CH2CH2)O + H2O → HO–(–CH2CH2–O–)n–H
Reactions with alcohols proceed similarly yielding ethylene glycol ethers:
- (CH2CH2)O + C2H5OH → HO–CH2CH2–OC2H5
- 2 (CH2CH2)O + C2H5OH → HO–CH2CH2–O–CH2CH2–OC2H5
Reactions with lower alcohols occur less actively than with water and require more severe conditions, such as heating to 160 °C and pressurizing to 3 MPa and adding an acid or alkali catalyst.
Reactions of ethylene oxide with fatty alcohols proceed in the presence of sodium metal, sodium hydroxide or boron trifluoride and are used for the synthesis of surfactants.[23]
Addition of carboxylic acids and their derivatives
Reactions, in presence of a catalyst, of ethylene oxide with carboxylic acids results in incomplete and with anhydrides in complete glycol ethers:
- (CH2CH2)O + CH3COOH → HO–CH2CH2–OCOCH3
- (CH2CH2)O + (CH3CO)2O → CH3COO–CH2CH2–OCOCH3
Similarly proceeds the addition of acid amides:
- (CH2CH2)O + CH3CONH2 → HO–CH2CH2–NHCOCH3
Addition of ethylene oxide to higher carboxylic acids is carried out at elevated temperatures (typically 140–180 °C) and pressure (0.3–0.5 MPa) in an inert atmosphere, in presence of an alkaline catalyst (concentration 0.01–2%), such as hydroxide or carbonate of sodium or potassium.[25] The carboxylate ion acts as nucleophile in the reaction:
- RCOOH + OH– → RCOO– + H2O
- (CH2CH2)O + RCOO– → RCOOCH2CH2O–
- RCOOCH2CH2O– + RCOOH → RCOOCH2CH2OH + RCOO–
Adding ammonia and amines
Ethylene oxide reacts with ammonia forming a mixture of mono-, di- and triethanolamine. The reaction is stimulated by adding a small amount of water.
- (CH2CH2)O + NH3 → HO–CH2CH2–NH2
- 2 (CH2CH2)O + NH3 → HO–CH2CH2)2NH2
- 3 (CH2CH2)O + NH3 → HO–CH2CH2)3N
Similarly proceed the reactions with primary and secondary amines:
- (CH2CH2)O + RNH2 → HO–CH2CH2–NHR
Dialkylamino ethanols can further react with ethylene oxide, forming amino polyethylene glycols:[7]
- n (CH2CH2)O + R2NCH2CH2OH → R2NCH2CH2O–(–CH2CH2O–)n–H
Trimethylamine reacts with ethylene oxide in the presence of water, forming choline:[26]
- (CH2CH2)O + (CH3)3N + H2O → [HOCH2CH2N (CH3)3]+OH–
Aromatic primary and secondary amines also react with ethylene oxide, forming the corresponding arylamino alcohols.
Halide addition
Ethylene oxide readily reacts with aqueous solutions of hydrochloric, hydrobromic and hydroiodic acids to form halohydrins. The reaction occurs easier with the last two acids:
- (CH2CH2)O + HCl → HO–CH2CH2–Cl
The reaction with these acids competes with the acid-catalyzed hydration of ethylene oxide; therefore, there is always a by-product of ethylene glycol with an admixture of diethylene glycol. For a cleaner product, the reaction is conducted in the gas phase or in an organic solvent.
Ethylene fluorohydrin is obtained differently, by boiling hydrogen fluoride with a 5–6% solution of ethylene oxide in diethyl ether. The ether normally has a water content of 1.5–2%; in absence of water, ethylene oxide polymerizes.[27]
Halohydrins can also be obtained by passing ethylene oxide through aqueous solutions of metal halides:[23]
- 2 (CH2CH2)O + CuCl2 + 2 H2O → 2 HO–CH2CH2–Cl + Cu(OH)2↓
Metalorganic addition
Interaction of ethylene oxide with organomagnesium compounds, which are Grignard reagents, can be regarded as nucleophilic substitution influenced by carbanion organometallic compounds. The final product of the reaction is a primary alcohol:
Similar mechanism is valid for other organometallic compounds, such as alkyl lithium:
Other addition reactions
Addition of hydrogen cyanide
Ethylene oxide easily reacts with the hydrogen cyanide forming ethylene cyanohydrin:
- (CH2CH2)O + HCN → HO–CH2CH2–CN
A slightly chilled (10–20 °C) aqueous solution of calcium cyanide can be used instead of HCN:[28]
- 2 (CH2CH2)O + Ca(CN)2 + 2 H2O → 2 HO–CH2CH2–CN + Ca(OH)2
Ethylene cyanohydrin easily loses water, producing acrylonitrile:
- HO–CH2CH2–CN → CH2=CH–CN + H2O
Addition of hydrogen sulfide and mercaptans
When reacting with the hydrogen sulfide, ethylene oxide forms 2-mercaptoethanol and thiodiglycol, and with alkylmercaptans it produces 2-alkyl mercaptoetanol:
- (CH2CH2)O + H2S → HO–CH2CH2–HS
- 2 (CH2CH2)O + H2S → (HO–CH2CH2)2S
- (CH2CH2)O + RHS → HO–CH2CH2–SR
The excess of ethylene oxide with an aqueous solution of hydrogen sulfide leads to the tris-(hydroxyethyl) sulfonyl hydroxide:
- 3 (CH2CH2)O + H2S → [(HO–CH2CH2)3S+]OH–
Addition of nitrous and nitric acids
Reaction of ethylene oxide with aqueous solutions of barium nitrite, calcium nitrite, magnesium nitrite, zinc nitrite or sodium nitrite leads to the formation of 2-nitroethanole:[29]
- 2 (CH2CH2)O + Ca(NO2)2 + 2 H2O → 2 HO–CH2CH2–NO2 + Ca(OH)2
With nitric acid, ethylene oxide forms mono- and dinitroglycols:[30]
Reaction with compounds containing active methylene groups
In the presence of alcoholates, reactions of ethylene oxide with compounds containing active methylene group leads to the formation of butyrolactones:[31]
Additions with aromatic compounds
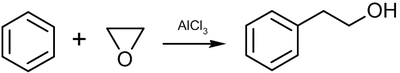
Ethylene oxide enters into the Friedel-Crafts reaction with benzene to form phenethyl alcohol:
Styrene can be obtained in one stage if this reaction is conducted at elevated temperatures (315–440 °C) and pressures (0.35–0.7 MPa), in presence of an aluminosilicate catalyst.[32]
Synthesis of crown ethers
A series of polynomial heterocyclic compounds, known as crown ethers, can be synthesized with ethylene oxide. One method is the cationic cyclopolymerization of ethylene oxide, limiting the size of the formed cycle:[33]
- n (CH2CH2)O → (–CH2CH2–O–)n
To suppress the formation of other linear polymers the reaction is carried out in a highly dilute solution.[33]
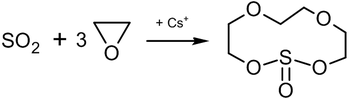
Reaction of ethylene oxide with sulfur dioxide in the presence of caesium salts leads to the formation of an 11-membered heterocyclic compound which has the complexing properties of crown ethers:[34]
Isomerization
When ethylene oxide is heated to about 400 °C, or to 150–300 °C in the presence of a catalyst (Al2O3, H3PO4, etc.), it isomerizes into acetaldehyde:[35]
The radical mechanism was proposed by Sidney W. Benson to explain this reaction in the gas phase; it comprises the following stages:[36]
1) (CH2CH2)O ↔ •CH2CH2O• → CH3CHO*
2) CH3CHO* → CH3• + CHO•
3) CH3CHO* + M → CH3CHO + M*
In reaction 3), M refers to the wall of the reaction vessel or to a heterogeneous catalyst. The moiety CH3CHO* represents a short-lived (lifetime of 10–8.5 seconds), activated molecule of acetaldehyde. Its excess energy is about 355.6 kJ/mol, which exceeds by 29.3 kJ/mol the binding energy of the C-C bond in acetaldehyde.[36]
In absence of a catalyst, the thermal isomerization of ethylene oxide is never selective and apart from acetaldehyde yields significant amount of by-products (see section Thermal decomposition).[37]
Reduction reaction
Ethylene oxide can be hydrogenated into ethanol in the presence of a catalyst, such as nickel, platinum, palladium,[37] boranes, lithium aluminium hydride and some other hydrides.[38].
Conversely, with some other catalysts, ethylene oxide may be reduced by hydrogen to ethylene with the yield up to 70%. The reduction catalysts include mixtures of zinc dust and acetic acid, of lithium aluminium hydride with titanium trichloride (the reducing agent is actually titanium dichloride, formed by the reaction between LiAlH4 and TiCl2) and of iron(III) chloride with butyllithium in tetrahydrofuran.[38]
Oxidation
Ethylene oxide can further be oxidized, depending on the conditions, to glycolic acid or carbon dioxide:
Deep gas-phase reactor oxidation of ethylene oxide at 800–1000 K and a pressure of 0.1–1 MPa yields a complex mixture of products containing O2, H2, CO, CO2, CH4, C2H2, C2H4, C2H6, C3H6, C3H8 and CH3CHO.[39]
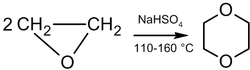
Dimerization
In the presence of acid catalysts, ethylene oxide can be dimerization into dioxane:
The reaction mechanism is as follows:[37]
The dimerization reaction is not selective, and there are always by-products, such as acetaldehyde (due to isomerization). The selectivity and speed of dimerization can be increased by adding a catalyst, such as platinum, platinum-palladium or iodine with sulfolan; however, 2-methyl-1,3-dioxolane is formed as a side product in the last case.[40]
Polymerization
Liquid ethylene oxide can form a polyethyleneglycols. The polymerization can proceeds via radical and ionic mechanisms, but only the latter has a wide practical application.[41] Cationic polymerization of ethylene oxide is assisted by protonic acids (HClO4, HCl), Lewis acids (SnCl4, BF3, etc.), organometallic compounds or more complex reagents:[41]
The reaction mechanism is as follows.[42] At the first stage, the catalyst (MXm) is initiated by alkyl-or acylhalogen or by compounds with active hydrogen atoms, usually water, alcohol or glycol:
- MXm + ROH → MXmRO–H+
The resulting active complex reacts with ethylene oxide via the SN2 mechanism:
- (CH2CH2)O + MXmRO–H+ → (CH2CH2)O•••H+O–RMXm
- (CH2CH2)O•••H+ O–RMXm → HO–CH2CH2+ + MXmRO–2
- HO–CH2CH2+ + n (CH2CH2)O → HO–CH2CH2–(O–CH2CH2)n+
The chain breaks as
- HO–CH2CH2–(O–CH2CH2)n+ + MXmRO– → HO–CH2CH2–(O–CH2CH2)n–OR + MXm
- H(O–CH2CH2)n–O–CH2–CH2+ + MXmRO– → H(O–CH2CH2)n–O–CH=CH2 + MXm + ROH
Anionic polymerization of ethylene oxide is assisted by bases, such as alcoholates hydroxides, carbonates or other compounds of alkali or alkaline earth metals.[41] The reaction mechanism is as follows:[42]
- (CH2CH2)O + RONa → RO–CH2CH2–O–Na+
- RO–CH2CH2–O–Na+ + n (CH2CH2)O → RO–(CH2CH2–O)n–CH2CH2–O–Na+
- RO–(CH2CH2–O)n–CH2CH2–O–Na+ → RO–(CH2CH2–O)n–CH=CH2 + NaOH
- RO–(CH2CH2–O)n–CH2CH2–O–Na+ + H2O → RO–(CH2CH2–O)(n+1)OH + NaOH
Thermal decomposition
Ethylene oxide is relatively stable to heating – in the absence of a catalyst, it does not dissociate up to 300 °C, and only above 570 °C there is a major exothermic decomposition, which proceeds through the radical mechanism.[37] The first stage involves isomerization, however high temperature accelerated the radical processes. They result in a gas mixture containing acetaldehyde, ethane, ethyl, methane, hydrogen, carbon dioxide, ketene and formaldehyde.[43] High-temperature pyrolysis (830–1200 K) at elevated pressure in an inert atmosphere leads to a more complex composition of the gas mixture, which also contains acetylene and propane.[44] Contrary to the isomerization, initiation of the chain occurs mainly as follows:[44]
- (CH2CH2)O → •CH2CH2O• → CH2O + CH2:
When carrying the thermal decomposition of ethylene oxide in the presence of transition metal compounds as catalysts, it is possible not only to reduce its temperature, but also to have ethyl as the main product, that is to reverse the ethylene oxide synthesis reaction.
Other reactions
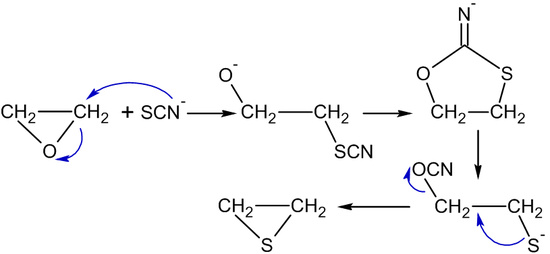
Thiocyanate ions or thiourea transform ethylene oxide into thiiranes (ethylene sulfides):[45]
- (CH2CH2)O + (NH2)2C=S → (CH2CH2)S + (NH2)2C=O
Reaction of phosphorus pentachloride with ethylene oxide produces ethylene dichloride:[23]
- (CH2CH2)O + PCl5 → Cl–CH2CH2–Cl + POCl3
Other dichloro derivatives of ethylene oxide can be obtained by combined action of sulfuryl chloride (SOCl2) and pyridine and of triphenylphosphine and carbon tetrachloride.[46]
Phosphorus trichloride reacts with ethylene oxide forming chloroethyl esters of phosphorous acid:[23]
- (CH2CH2)O + PCl3 → Cl–CH2CH2–OPCl2
- 2 (CH2CH2)O + PCl3 → (Cl–CH2CH2–O)2PCl
- 3 (CH2CH2)O + PCl3 → Cl–CH2CH2–O)3P
The reaction product of ethylene oxide with acyl chlorides in the presence of sodium iodide is a complex iodoethyl ether:[46]
- (CH2CH2)O + RCOCl + NaI → RC(O)–OCH2CH2–I + NaCl
Heating ethylene oxide to 100 °C with carbon dioxide, in a non-polar solvent in the presence of bis-(triphenylphosphine)-nickel(0) results in ethylene carbonate:[47]
In industry, a similar reaction is carried out at high pressure and temperature in the presence of quaternary ammonium or phosphonium salts as a catalyst.[48]
Reaction of ethylene oxide with formaldehyde at 80–150 °C in the presence of a catalyst leads to the formation of 1,3-dioxolane:[49]
Substituting formaldehyde by other aldehydes or ketones results in a 2-substituted 1,3-dioxolane (yield: 70–85%, catalyst: tetraetilammoniybromid).[49]
Catalytic hydroformylation of ethylene oxide results in hydroxypropanal and further in propane-1,3-diol:[50]
Laboratory synthesis
Dehydrochlorination of ethylene and its derivatives
Dehydrochlorination of 2-chloroethanol, developed by Wurtz back in 1859, still remains one of the most common laboratory methods of producing ethylene oxide:
- Cl–CH2CH2–OH + NaOH → (CH2CH2)O + NaCl + H2O
The reaction is carried out at elevated temperature, and beside sodium hydroxide or potassium hydroxide, calcium hydroxide, barium hydroxide, magnesium hydroxide or carbonates of alkali or alkaline earth metals can be used.[51]
Chloroethanol, in turn, is synthesized using one of the following methods:[51]
By reacting ethylene glycol hydrochloric acid:
- OH–CH2CH2–OH + HCl → OH–CH2CH2–Cl + H2O
By reacting ethylene with hypochlorous acid:
- CH2=CH2 + HOCl → OH–CH2CH2–Cl
or by chlorination of ethylene
- CH2=CH2 + Cl2 + H2O → OH–CH2CH2–Cl + HCl
Another convenient and old method of ethylene oxide synthesis is reaction of an alkali with chloroethyl acetate:[52]
- Cl–CH2CH2–OCOCH3 + 2 KOH → (CH2CH2)O + KCl + CH3COOK + H2O
With a high yield (90%) ethylene oxide can be produced by reacting calcium oxide with ethyl hypochlorite; substituting calcium by other alkaline earth metals reduces the reaction yield:[52]
- 2 CH3CH2–OCl + CaO → 2 (CH2CH2)O + CaCl2 + H2O
In turn, ethylhypochlorite is synthesized as follows:
- Cl2 + NaOH + CH3CH2OH → CH3CH2OCl + NaCl + H2O
Direct oxidation of ethylene by peroxy acids
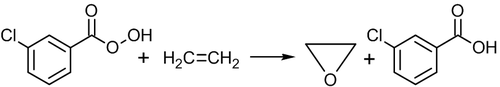
Ethylene can be directly oxidized into ethylene oxide using peroxy acids, for example, peroxybenzoic or meta-chloro-peroxybenzoic acid:[53]
Oxidation by peroxy acids is efficient for higher alkenes, but not for ethylene. The above reaction is slow and has low yield, therefore it is not used in the industry.[52]
Other preparative methods
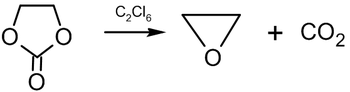
Other synthesis methods include[52] reaction of diiodo ethane with silver oxide:
- I–CH2CH2–I + Ag2O → (CH2CH2)O + 2 AgI
and decomposition of ethylene carbonate at 200–210 °C in the presence of hexachloroethane:
Industrial synthesis
History
Commercial production of ethylene oxide dates back to 1914 when BASF built the first factory which used the chlorohydrin process (reaction of ethylene chlorohydrin with calcium hydroxide). The chlorohydrin process was unattractive for several reasons, including low efficiency and loss of valuable chlorine into calcium chloride.[54] More efficient direct oxidation of ethylene by air was invented by Lefort in 1931 and in 1937 Union Carbide opened the first plant using this process. It was further improved in 1958 by Shell Oil Co. by replacing air with oxygen and using elevated temperature of 200–300 °C and pressure (1–3 MPa).[55] This more efficient routine accounted for about half of ethylene oxide production in the 1950s in the U.S., and after 1975 it completely replaced the previous methods.[55]
Chlorohydrin process of production of ethylene oxide
Although the chlorohydrin process is almost entirely superseded in the industry by the direct oxidation of ethylene, the knowledge of this method is still important for educational reasons and because it is still used in the production of propylene oxide.[56] The process consists of three major steps: synthesis of ethylene chlorohydrin, dehydrochlorination of ethylene chlorohydrin to ethylene oxide and purification of ethylene oxide. Those steps are carried continuously. In the first column, hypochlorination of ethylene is carried out as follows:[57]
- Cl2 + H2O → HOCl + HCl
- CH2=CH2 + HOCl → OH–CH2CH2–Cl
- CH2=CH2 + Cl2 → Cl–CH2CH2–Cl
To suppress the conversion of ethylene into the ethylene dichloride (the last reaction), the concentration of ethylene is maintained at about 4–6%, and the solution is heated by steam to the boiling point.[57]
Next, aqueous solution of ethylene chlorohydrin enters the second column, where it reacts with a 30% solution of calcium hydroxide at 100 °C:[57]
- 2 OH–CH2CH2–Cl + Ca(OH)2 → 2 (CH2CH2)O + CaCl2 + H2O
The produced ethylene oxide is purified by rectification. The chlorohydrin process allows to reach 95% conversion of ethylene chlorohydrin. The yield of ethylene oxide is about 80% of the theoretical value; for 1 ton of ethylene oxide, about 200 kg of ethylene dichloride is produced.[57]
Direct oxidation of ethylene
Usage in the global industry
Direct oxidation of ethylene was patented by Lefort in 1931. This method was repeatedly modified for industrial use, and at least four major variations are known. They all use oxidation by oxygen or air and a silver-based catalyst, but differ in the technological details and hardware implementations.[58]
Union Carbide (currently a division of Dow Chemical Company) was the first company to develop the direct oxidation process. Since 1994, it uses the so-called METEOR process (Most Effective Technology for Ethylene Oxide Reactions) which is characterized by high productivity, low initial capital investment and low operating costs. The method is the exclusive property of the company; it is used only at its own plants and accounts for about 20% of the global ethylene oxide production.[59]
A similar production method was developed by Scientific Design Co., but it received wider use because of the licensing system – it accounts for 25% of the world's production and for 75% of world's licensed production of ethylene oxide.[59][60] A proprietary variation of this method is used by Japan Catalytic Chemical Co., which adapted synthesis of both ethylene oxide and ethylene glycol in a single industrial complex.
A different modification was developed Shell International Chemicals BV. Their method is rather flexible with regard to the specific requirements of specific industries; it is characterized by high selectivity with respect to the ethylene oxide product and long lifetime of the catalyst (3 years). It accounts for about 40% of global production.[59]
Older factories typically use air for oxidation whereas newer plants and processes, such as METEOR and Japan Catalytic, favor oxygen.[61]
Chemistry and kinetics of the direct oxidation process
Formally, the direct oxidation process is expressed by the following equation:
However, significant yield of carbon dioxide and water is observed in practice, which can be explained by the complete oxidation of ethylene or ethylene oxide:
- CH2=CH2 + 3 O2 → 2 CO2 + 2 H2O
- 2 (CH2CH2)O + 5 O2 → 4 CO2 + 4 H2O
The process of heterogeneous catalytic oxidation of ethylene was studied by P. A. Kilty and W. M. H. Sachtler, who suggested the following mechanism:[62]
- O2 + 4 Ag(adj) → 4 Ag + 2 O2–(ads)
- O2 + Ag → Ag+ + O2–
- O2–(ads) + CH2=CH2 → (CH2CH2)O + O(ads)
- 6 O (ads) + CH2=CH2 → 2 CO2 + 2 H2O
Here (ads) refers to particles adsorbed on the catalyst surface and (adj) to particles of silver, directly adjacent to the oxygen atoms.
Thus the overall reaction is expressed as
- 7 CH2=CH2 + 6 O2 → 6 (CH2CH2)O + 2 CO2 + 2 H2O
and the maximum degree of conversion of ethylene to ethylene oxide is 6/7 or 85.7%.[62]
The catalyst for the reaction is metallic silver deposited on various matrixes, including pumice, silica gel, various silicates and aluminosilicates, alumina and silicon carbide, and activated by certain additives (antimony, bismuth, barium peroxide, etc.).[63] The process temperature was optimized as 220–280 °C. Lower temperatures reduce the activity of the catalyst, and higher temperatures promote the complete oxidation of ethylene thereby reducing the yield of ethylene oxide. Elevated pressure of 1–3 MPa increases the productivity of the catalyst and facilitates absorption of ethylene oxide from the reacting gases.[63]
Whereas oxidation by air is still being used, oxygen (> 95% purity) is preferred for several reasons, such as higher molar yield of ethylene oxide (75–82% for oxygen vs. 63–75% for air), higher reaction rate (no gas dilution) and no need of separating nitrogen in the reaction products.[7][64]
World production of ethylene oxide
The world production of ethylene oxide was 19 million tonnes in 2008 and 18 million tonnes in 2007.[65] This places ethylene oxide 14th most produced organic chemical, whereas the most produced one was ethylene with 113 million tonnes.[66] SRI Consulting forecasted the growth of consumption of ethylene oxide of 4.4% per year during 2008–2013 and 3% from 2013 to 2018.[65]
In 2004, the global production of ethylene oxide by region was as follows:[67]
| Region | Number of major producers | Production, thousand tonnes |
|---|---|---|
| North America United States Canada Mexico |
10 3 3 |
4009 1084 350 |
| South America Brazil Venezuela |
2 1 |
312 1982 |
| Europe Belgium France Germany Netherlands Spain Turkey United Kingdom Eastern Europe |
2 1 4 2 1 1 1 no data |
770 215 995 460 100 115 300 950 |
| Middle East Iran Kuwait Saudi Arabia |
2 1 2 |
201 350 1781 |
| Asia China Taiwan India Indonesia Japan Malaysia South Korea Singapore |
No data 4 > 2 1 4 1 3 1 |
1354 820 488 175 949 385 740 80 |
The world's largest producers of ethylene oxide are Dow Chemical Company (3–3.5 million tonnes in 2006[68]), Saudi Basic Industries (2000–2500 tonnes in 2006[68]), Royal Dutch Shell (1.328 million tonnes in 2008–2009[69][70][71][72][73]), BASF (1.175 million tonnes in 2008–2009[74]), China Petrochemical Corporation (~1000 tonnes in 2006[68]), Formosa Plastics (~1 million tonnes in 2006[68]) and Ineos (0.92 million tonnes in 2008–2009).[75]
Applications

Ethylene oxide is one of the most important raw materials used in the large-scale chemical production. Most ethylene oxide is used for synthesis of ethylene glycols, including diethylene glycol and triethylene glycol, that accounts for up to 75% of global consumption. Other important products include ethylene glycol ethers, ethanolamines and ethoxylates. Among glycols, ethylene glycol is used as antifreeze, in the production of polyester and polyethylene terephthalate (PET – raw material for plastic bottles), liquid coolants and solvents. Polyethyleneglycols are used in perfumes, cosmetics, pharmaceuticals, lubricants, paint thinners and plasticizers. Ethylene glycol ethers are part of brake fluids, detergents, solvents, lacquers and paints. Other products of ethylene oxide. Ethanolamines are used in the manufacture of soap and detergents and for purification of natural gas. Ethoxylates are reaction products of ethylene oxide with higher alcohols, acids or amines. They are used in the manufacture of detergents, surfactants, emulsifiers and dispersants.[76]
Whereas synthesis of ethylene glycols is the major application of ethylene oxide, its percentage varies greatly depending on the region: from 44% in the Western Europe, 63% in Japan and 73% in North America to 90% in the rest of Asia and 99% in Africa.[77]
Production of ethylene glycol
Ethylene glycol is industrially produced by non-catalytic hydration of ethylene oxide at a temperature of 200 °C and a pressure of 1.5–2 MPa:[78]
- (CH2CH2)O + H2O → HOCH2CH2OH
By-products of the reaction are diethylene glycol, triethylene glycol and polyglycols with the total of about 10%, which are separated from the ethylene glycol by distillation at reduced pressure.[79]
Another synthesis method is the reaction of ethylene oxide and CO2 (temperature 80–120 °C and pressure of 5.2 MPa) yielding ethylene carbonate and its subsequent hydrolysis with decarboxylation:[78]
Modern technologies of production of ethylene glycol include the following.[80] Shell OMEGA technology (Only Mono-Ethylene Glycol Advantage) is a two-step synthesis of ethylene carbonate using a phosphonium halide as a catalyst. The glycol yield is 99–99.5%, with other glycols practically absent. The main advantage of the process is production of pure ethylene glycol without the need for further purification. The first commercial plant which uses this method was opened in 2008 in South Korea.[81] Dow METEOR (Most Effective Technology for Ethylene Oxide Reactions) is an integrated technology for producing ethylene oxide and its subsequent hydrolysis into ethylene glycol. The glycol yield is 90–93%. The main advantage of the process is relative simplicity, using fewer stages and less equipment.
Production of glycol ethers
The major industrial esters of mono-, di- and triethylene glycols are methyl, ethyl and normal butyl ethers, as well as their acetates and phthalates. The synthesis involves reaction of the appropriate alcohol with ethylene oxide:[82]
- (CH2CH2)O + ROH → HOCH2CH2OR
- (CH2CH2)O + HOCH2CH2OR → HOCH2CH2OCH2CH2OR
- (CH2CH2)O + HOCH2CH2OCH2CH2OR → HOCH2CH2OCH2CH2OCH2CH2OR
The reaction of monoesters with an acid or its anhydride leads to the formation of the esters:
- CH3COOH + HOCH2CH2OR → ROCH2CH2OCOCH3 + H2O
Production of ethanolamines
In the industry, ethanolamines (mono-, di- and triethanolamines) are produced by reacting ammonia and ethylene oxide in anhydrous medium at a temperature of 40–70 °C and pressure of 1.5–3.5 MPa:[83]
- CH2CH2)O + NH3 → HOCH2CH2NH2
- 2 (CH2CH2)O + NH3 → (HOCH2CH2)2NH
- 3 (CH2CH2)O + NH3 → (HOCH2CH2)3N
All three ethanolamines are produced in the process, while ammonia and part of methylamine are recycled. The final products are separated by vacuum distillation. Hydroxyalkylamines are produced in a similar process:
- CH2CH2)O + RNH2 → HOCH2CH2NHR
- 2 (CH2CH2)O + RNH2 → (HOCH2CH2)2NR
Monosubstituted products are formed by reacting a large excess of amine with ethylene oxide in presence of water and at a temperature below 100 °C. Disubstituted products are obtained with a small excess of ethylene oxide, at a temperature of 120–140 °C and a pressure of 0.3–0.5 MPa.[84]
Production of ethoxylates
Industrial production of ethoxylates is realized by a direct reaction of higher alcohols, acids or amines with ethylene oxide in the presence of an alkaline catalyst at a temperature of 120–180 °C. Modern plants producing ethoxylates are usually based on the BUSS LOOP reactors technology,[85] which is based on a three-stage continuous process. In the first stage, the initiator or catalyst of the reaction and the feedstock are fed into the container, where they are mixed, heated and vacuum dried. Then reaction is carried out in a special insulated reactor in an inert atmosphere (nitrogen) to prevent a possible explosion of ethylene oxide. Finally, the reaction mixture is neutralized, degassed and purified.[86]
Production of acrylonitrile
Currently, most acrylonitrile (90% in 2008) is produced by the SOHIO method, which is based on the catalytic oxidation of propylene in the presence of ammonia and bismuth phosphomolybdate. However, until 1960 a key production process was addition of hydrogen cyanide to ethylene oxide, followed by dehydration of the resulting cyanohydrin:[87][88]:
Addition of hydrocyanic acid to ethylene oxide is carried out in the presence of a catalyst (sodium hydroxide and diethylamine), and dehydration of cyanohydrin occurs in the gas phase upon the catalytic action of aluminium oxide.[89]
Other uses
The direct use of ethylene oxide accounts for only 0.05% (2004 data) of its global production.[67] Ethylene oxide is used as a fumigant and disinfecting agent, as a mixture with carbon dioxide (8.5–80% of ethylene oxide), nitrogen or dichlorodifluoromethane (12% ethylene oxide). It is applied for gas-phase sterilization of medical equipment and instruments, packaging materials and clothing, surgical and scientific equipment;[67] for processing of storage facilities (tobacco, packages of grain, sacks of rice, etc.), clothing, furs and valuable documents.[90]
Ethylene oxide is also used as a flame retardant, accelerator of maturation of tobacco leaves and fungicide.[90] Ethylene oxide is also used as a main component of thermobaric weapons (fuel-air explosives).[2][3][91]
Identification of ethylene oxide
The simplest qualitative reaction for ethylene oxide uses its property to precipitate insoluble hydroxides of metals when it is passed through aqueous solutions of their salts, for example
- 2 (CH2CH2)O + MnCl2 + 2 H2O → 2 HO–CH2CH2–Cl + Mn(OH)2↓
Similarly, ethylene oxide is detected by the bright pink color of the indicator when passing air through aqueous solutions of some salts of sodium or potassium (chlorides, iodides, thiosulfates, etc.) with the addition of phenolphthalein:[92]
- (CH2CH2)O + NaCl + H2O → HO–CH2CH2–Cl + NaOH
Other methods of ethylene oxide detection are[92] color reactions with pyridine derivatives and hydrolysis of ethylene glycol with periodic acid. The produced iodic acid is detected with silver nitrate.
The main physical method of ethylene oxide detection is gas chromatography.[67]
Fire and fire hazards
Ethylene oxide is extremely flammable and its mixtures with air are explosive. When heated, it may rapidly expand causing fire and explosion.[93] The autoignition temperature is 429 °C, minimum inflammable content in the air is 2.7%,[94] and the NPFA rating is NFPA 704.[95]
Fires caused by ethylene oxide are extinguished by traditional media, including foam, carbon dioxide or water. Extinguishing of burning ethylene oxide is complicated by that it can continue burning in an inert atmosphere and in water solutions. Fire suppression is reached only upon dilution with water above 22:1.[96]
Physiological effects
Effect on microorganisms
Ethylene oxide inhibits growth of microorganisms (disinfectant properties) and when present in high concentrations, can completely destroy them. Strong alkylating properties make ethylene oxide a universal poison for protoplasm: it causes clotting of proteins, deactivation of enzymes and other biologically important components of a living organism.[97]
Ethylene oxide acts stronger against bacteria, especially gram-positive bacteria, than against the yeast and fungi.[97] Disinfecting effect of ethylene oxide is similar to sterilization by heat, but because of limited penetration, its affects only the surface. The Sterility Assurance Level, after a certain specified exposure to ethylene oxide is 10−6 meaning the chance of finding a bacteria of below 1 per million.[98]
Effects on humans and animals
Ethylene oxide is an alkylating agent; it has irritating, sensitizing and narcotic effects.[99] Chronic exposure to ethylene oxide also induces mutagenic effects. The International Agency for Research on Cancer classifies ethylene oxide into group 1, meaning it is a proven carcinogen.[100][101] A 2003 study of 7,576 women exposed while at work in commercial sterilization facilities in the U.S. suggests ethylene oxide is associated with breast cancer incidence.[102] A 2004 follow up study analyzing 18,235 men and women workers exposed to ethylene oxide from 1987 to 1998 concluded "There was little evidence of any excess cancer mortality for the cohort as a whole, with the exception of bone cancer based on small numbers. Positive exposure-response trends for lymphoid tumors were found for males only. Reasons for the sex specificity of this effect are not known. There was also some evidence of a positive exposure-response for breast cancer mortality."[103]
Ethylene oxide is toxic by inhalation with an U.S. OSHA permissible exposure limit of 1 ppm calculated as a time weighted average (TWA) over 8 hours of 1 ppm, and a short term exposure limit (excursion limit) calculated as a TWA over 15 minutes of 5 ppm. [29 CFR 19101.1048]. At concentrations in the air about 200 parts per million, ethylene oxide irritates mucous membranes of the nose and throat; higher contents cause damage to the trachea and bronchi, progressing into the partial collapse of the lungs. High concentrations can cause pulmonary edema and damage the cardiovascular system; the damaging effect of ethylene oxide may occur only after 72 hours after exposure.[15] The maximum content of ethylene oxide in the air according to the U.S. standards (ACGIH) is 1.8 mg/m3.[104] NIOSH has determined that the Immediately Dangerous to Life and Health level (IDLH) is 800 ppm.[105] Because the odor threshold for ethylene oxide varies between 250 and 700 ppm, it is imperceptible to human until well above dangerous levels. Therefore continuous monitors are standard practice. Currently, the substance is banned from the use for plant protection by the EU government.[106]
Ethylene oxide causes acute poisoning, accompanied by the following symptoms: slight heartbeat, muscle twitching, flushing, headache, diminished hearing, acidosis, vomiting, dizziness, transient loss of consciousness and a sweet taste in the mouth. Acute intoxication is accompanied by a strong throbbing headache, dizziness, difficulty in speech and walking, sleep disturbance, pain in the legs, weakness, stiffness, sweating, increased muscular irritability, transient spasm of retinal vessels, enlargement of the liver and suppression of its antitoxic functions.[99]
Ethylene easily penetrates through the clothing and footwear, causing skin irritation and dermatitis with the formation of blisters, fever and leukocytosis.[99]
The median lethal doses (LD50, or a dose required to kill half the members of a tested population after a certain time) for ethylene oxide are 72 mg/kg (rat, oral) and 187 mg/kg (rat, subcutaneous injection).[107]
References
- ↑ John J. McKetta, William A. Cunningham (1984). Encyclopedia of Chemical Processing and Design. 20. CRC Press. p. 309. ISBN 0824724704. http://books.google.com/?id=oJy5wdzi0yUC&pg=PA309.
- ↑ 2.0 2.1 Eric Croddy, James J. Wirtz (2005). Weapons of mass destruction: an encyclopedia of worldwide policy, technology, and history, Volume 2. ABC-CLIO. p. 136. ISBN 1851094903. http://books.google.com/?id=ZzlNgS70OHAC&pg=PA136.
- ↑ 3.0 3.1 Rudolf Meyer, Josef Köhler, Axel Homburg (2007). Explosives. Wiley-VCH. p. 142. ISBN 3527316566. http://books.google.com/?id=ATiYCfo1VcEC&pg=PA142.
- ↑ Siegfried Rebsdat, Dieter Mayer "Ethylene Oxide" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a10_117.
- ↑ Wurtz, A. (1859). Compt. Rend. 48: 101–104.
- ↑ 6.0 6.1 6.2 PV Zimakova and Ph. O. Dymenta, ed (1967). "Part I. Structure and properties of ethylene oxide. Features of the reactivity of ethylene oxide and the structure of its molecules". Ethylene oxide. Khimiya. pp. 15–17.
- ↑ 7.0 7.1 7.2 7.3 7.4 "Etylene Oxide". Kirk-Othmer Encyclopedia of Chemical Technology. Elastomers, synthetic to Expert Systems. 9 (4 ed.). New York: John Wiley & Sons. 1994. pp. 450–466.
- ↑ Lefort, T.E. (1935). "Process for the production of ethylene oxide. United States Patent 1998878". http://www.freepatentsonline.com/1998878.pdf. Retrieved 2009-09-23.
- ↑ P. P. McClellan (1950). "Manufacture and Uses of Ethylene Oxide and Ethylene Glycol". Ind. Eng. Chem. 42: 2402–2407. doi:10.1021/ie50492a013.
- ↑ Knunyants, IL, ed (1988). "Voltage molecules". Chemical Encyclopedia. 3. "Soviet encyclopedia". pp. 330–334.
- ↑ 11.0 11.1 Traven VF (2004). VFTraven. ed. Organic chemistry: textbook for schools. 2. ECC "Academkniga". pp. 102–106. ISBN 5946281720.
- ↑ Cunningham G. L., Levan W. I., Gwinn W. D. (1948). "The Rotational Spectrum of Ethylene Oxide". Phys. Rev. 74: 1537. doi:10.1103/PhysRev.74.1537.
- ↑ "The dipole moments of certain substances". ChemAnalitica.com. 1 April 2009. http://chemanalytica.com/book/novyy_spravochnik_khimika_i_tekhnologa/12_obshchie_svedeniya/6106. Retrieved 2009-09-21.
- ↑ Kondrat'ev, VN, ed (1974). Energy of chemical bonds. Ionization potentials and electron affinity. Nauka. pp. 77–78.
- ↑ 15.0 15.1 "Medical Management Guidelines for Ethylene Oxide". Medical Management Guidelines (MMGs). Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/MHMI/mmg137.html. Retrieved 2009-09-29.
- ↑ "Ethylene oxide" (in Russian). Great Soviet Encyclopedia. http://slovari.yandex.ru/~%D0%BA%D0%BD%D0%B8%D0%B3%D0%B8/%D0%91%D0%A1%D0%AD/%D0%AD%D1%82%D0%B8%D0%BB%D0%B5%D0%BD%D0%B0%20%D0%BE%D0%BA%D0%B8%D1%81%D1%8C/. Retrieved 2009-09-25.
- ↑ "Термодинамические показатели органических соединений". ChemAnalitica.com. 1 April 2009 года. http://chemanalytica.com/book/novyy_spravochnik_khimika_i_tekhnologa/12_obshchie_svedeniya/6084. Retrieved 2009-09-21.
- ↑ Knunyants, I. L., ed (1988). "Ethylene oxide". Chemical encyclopedia. 5. pp. 990–991.
- ↑ "Surface tension of liquefied gas at the border with its own steam". ChemAnalitica.com. 1 April 2009. http://chemanalytica.com/book/novyy_spravochnik_khimika_i_tekhnologa/12_obshchie_svedeniya/6118. Retrieved 2009-09-21.
- ↑ "boiling point or sublimation (°C) organic matter in the vapor pressure above 101.3 kPa". ChemAnalitica.com. 1 April 2009. http://chemanalytica.com/book/novyy_spravochnik_khimika_i_tekhnologa/12_obshchie_svedeniya/6061. Retrieved 2009-09-21.
- ↑ "viscosity of organic compounds". ChemAnalitica.com. 1 April 2009. http://chemanalytica.com/book/novyy_spravochnik_khimika_i_tekhnologa/12_obshchie_svedeniya/6112. Retrieved 2009-09-21.
- ↑ "vapor pressure of organic compounds". ChemAnalitica.com. 1 April 2009. http://chemanalytica.com/book/novyy_spravochnik_khimika_i_tekhnologa/12_obshchie_svedeniya/6063. Retrieved 2009-09-21.
- ↑ 23.0 23.1 23.2 23.3 23.4 PV Zimakova and Mr. O. Dymenta, ed (1967). "Chapter III. Review of the individual reactions of ethylene oxide". Ethylene oxide. M.: Khimiya. pp. 90–120.
- ↑ "Epoxyethane (Ethylene Oxide)". Alkenes menu. Chemguide. http://www.chemguide.co.uk/organicprops/alkenes/epoxyethane.html. Retrieved 2009-10-05.
- ↑ N. M. van Os, ed (1998). Nonionic surfactants: organic chemistry. CRC Press. pp. 129–131. ISBN 9780824799977. http://books.google.com/?id=YoZ6CjYNLoQC&pg=PA129.
- ↑ Petrov, AA, Balian HV, Troshchenko AT (2002). "Chapter 12. Amino alcohol". In Stadnichuk. Organic chemistry (5 ed.). St. Petersburg.. p. 286. ISBN 5819400674.
- ↑ William A. Sheppard, Clay M. Sharts (1969). Organic Fluorine Chemistry. W.A. Benjamin. p. 98. ISBN 0805387900.
- ↑ "Ethylene cyanohydrin". Organic Syntheses 1: 256. 1941. http://www.orgsyn.org/orgsyn/pdfs/cv1p0256.pdf.
- ↑ "2-Nitroethanol". Organic Syntheses 5: 833. 1973. http://www.orgsyn.org/orgsyn/pdfs/CV5P0833.pdf.
- ↑ Orlova, EY (1981). Chemistry and technology of high explosives: Textbook for high schools (3 ed.). Khimiya. p. 278.
- ↑ Vogel A.I. (1989). Vogel's Textbook of practical organic chemistry (5 ed.). UK: Longman Scientific & Techical. p. 1088. ISBN 0582462363.
- ↑ "United States Patent 4443643. Reaction of benzene with ethylene oxide to produce styrene". http://www.freepatentsonline.com/4443643.pdf. Retrieved 2009-10-13.
- ↑ 33.0 33.1 Hiraoka M. (1982). Crown Compounds. Their Characteristics and Applications. Kodansha. pp. 33–34. ISBN 4061394444.
- ↑ Roesky H. W., Schmidt H. G. (1985). "Reaction of Ethylene Oxide with Sulfur Dioxide in the Presence of Cesium Ions: Synthesis of 1,3,6,9,2 λ 4-Tetraoxathia-2-cycloundecanone". Angewandte Chemie International Edition 24: 695. doi:10.1002/anie.198506951.
- ↑ Petrov, AA, Balian HV, Troshchenko AT (2002). "Chapter 4. Ethers". Organic chemistry (5 ed.). St. Petersburg.. pp. 159–160. ISBN 5819400674.
- ↑ 36.0 36.1 Benson S. W. (1964). "Pyrolysis of Ethylene Oxide. A Hot Molecule Reaction". The Journal of Chemical Physics 40: 105. doi:10.1063/1.1729851.
- ↑ 37.0 37.1 37.2 37.3 PV Zimakova and Mr. O. Dymenta, ed (1967). "Chapter II. Chemical properties of ethylene oxide". Ethylene oxide. Khimiya. pp. 57–85.
- ↑ 38.0 38.1 Hudlický M. (1984). Reductions in Organic Chemistry. Chichester: Ellis Horwood Limited. p. 83. ISBN 0853123454.
- ↑ Dagaut P., Voisin D., Cathonnet M., Mcguinness M., Simmie J. M. (1996). "The oxidation of ethylene oxide in a jet-stirred reactor and its ignition in shock waves". Combustion and Flame 156: 62–68.
- ↑ "United States Patent 3998848. Cyclodimerization of ethylene oxide". http://www.freepatentsonline.com/3998848.pdf.
- ↑ 41.0 41.1 41.2 Dyment, ON, Kazanskii, KS, Miroshnikov AM (1976). ON Dymenta. ed. glycol and other derivatives of ethylene oxide and propylene. Khimiya. pp. 214–217.
- ↑ 42.0 42.1 Joseph C. Salamone, ed (1996). Polymeric materials encyclopedia. 8. CRC Press. pp. 6036–6037. ISBN 9780849324703.
- ↑ Neufeld L.M., Blades A.T. (1963). "The Kinetics of the Thermal Reactions of Ethylene Oxide". Canadian Journal of Chemistry 41: 2956. doi:10.1139/v63-434. http://article.pubs.nrc-cnrc.gc.ca/ppv/RPViewDoc?issn=1480-3291&volume=41&issue=12&startPage=2956.
- ↑ 44.0 44.1 Lifshitz A., Ben-Hamou H. (1983). "Thermal reactions of cyclic ethers at high temperatures. 1. Pyrolysis of ethylene oxide behind reflected shocks". The Journal of Physical Chemistry 87: 1782. doi:10.1021/j100233a026.
- ↑ Gilchrist T. (1985). Heterocyclic Chemistry. Pearson Education. pp. 411–412. ISBN 8131707938.
- ↑ 46.0 46.1 Michael Smith, Michael B. Smith, Jerry March (2007). Advanced organic chemistry. Reactions, Mechanisms and Structure. Wiley-Interscience. ISBN 0471720917. http://books.google.com/?id=JDR-nZpojeEC.
- ↑ L. Fieser, M. Fieser (1979). Reagents for Organic Synthesis. 7. Wiley. p. 545. ISBN 9780471029182.
- ↑ Sheldon RA (1983). Chemicals from synthesis gas: catalytic reactions of CO and, Volume 2. Springer. p. 193. ISBN 9027714894. http://books.google.com/?id=s1_rjRUlu1EC&pg=PA193.
- ↑ 49.0 49.1 L. Fieser, M. Fieser (1977). Reagents for Organic Synthesis. 6. Wiley. p. 197. ISBN 9780471258735.
- ↑ "United States Patent 20030032845. Hydroformylation of ethylene oxide". http://www.freepatentsonline.com/20030032845.pdf.
- ↑ 51.0 51.1 PV Zimakova and O. Dymenta, ed (1967). "Chapter V. Producing ethylene oxide through ethylene". Ethylene oxide. Khimiya. pp. 155–182.
- ↑ 52.0 52.1 52.2 52.3 PV Zimakova and Mr. O. Dymenta, ed (1967). "Part II. Synthesis of ethylene oxide. Overview of reactions of formation of ethylene oxide and other α-oxides". Ethylene oxide. Khimiya. pp. 145–153.
- ↑ McMurry J. (2008). Organic chemistry (7 ed.). Thomson. p. 661. ISBN 0495112585.
- ↑ Norris J.F. (1919). "The Manufacture of War Gases in Germany". Journal of Industrial and Engineering Chemistry 11: 817.
- ↑ 55.0 55.1 Weissermel K., Arpe H-J. (2003). Industrial organic chemistry (4 ed.). Weinheim: Wiley-VCH. pp. 145–148. ISBN 9783527305780.
- ↑ "Process Economics Program Report 2D". PEP Report. SRI Consulting. February 1985. http://www.sriconsulting.com/PEP/Public/Reports/Phase_84/RP002D/. Retrieved 2009-11-19.
- ↑ 57.0 57.1 57.2 57.3 Yukelson II (1968). The technology of basic organic synthesis. Khimiya. pp. 554–559.
- ↑ D.D. Eley, H. Pines, P.B. Weisz, ed (1967). "Catalitic Oxidation of Olefins". Advances in catalysis and related subjects. 17. New York: Academic Press Inc. pp. 156–157.
- ↑ 59.0 59.1 59.2 Bloch H. P., Godse A. (2006). Compressors and modern process applications. John Wiley and Sons. pp. 295–296. ISBN 9780471727927.
- ↑ "Ethylene Oxide/Ethylene Glycol Process". Process Licensing and Engineering. Scientific Design Company. http://www.scidesign.com/Business/EO%20-%20EG%20Process/EO_EG_Process.htm. Retrieved 2009-10-03.
- ↑ Chauvel A., Lefebvre G. (1989). Petrochemical processes 2. Major Oxygenated, Chlorinated and Nitrated Derivatives. 2 (2 ed.). Paris: Editions Technip. p. 4. ISBN 2710805634.
- ↑ 62.0 62.1 Kilty P. A., Sachtler W. M. H. (1974). "The mechanism of the selective oxidation of ethylene to ethylene oxide". Catalysis Reviews: Science and Engineering 10: 1–16. doi:10.1080/01614947408079624.
- ↑ 63.0 63.1 Lebedev, N.N.. Chemistry and technology of basic organic and petrochemical synthesis (4 ed.). Khimiya. pp. 420–424. ISBN 5724500086.
- ↑ Gunardson H. (1998). Industrial gases in petrochemical processing. New York: Marcel Dekker, Inc.. pp. 131–132. ISBN 0824799089.
- ↑ 65.0 65.1 "Ethylene Oxide". WP Report. SRI Consulting. January 2009. http://www.sriconsulting.com/WP/Public/Reports/eo/. Retrieved 2009-09-29.
- ↑ "Ethylene". WP Report. SRI Consulting. January 2009. http://www.sriconsulting.com/WP/Public/Reports/ethylene/. Retrieved 2009-09-29.
- ↑ 67.0 67.1 67.2 67.3 67.4 1,3-Butadiene, Ethylene Oxide and Vinyl Halides (Vinyl Fluoride, Vinyl Chloride and Vinyl Bromide) (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 97 ed.). Lyon: International Agency for Research on Cancer. 2008. pp. 185–287. ISBN 9789283212973.
- ↑ 68.0 68.1 68.2 68.3 Devanney M. T. (April 2007). 2009/RP2I/ "Ethylene Oxide". SEH Peport. SRI Consulting. http://www.sriconsulting.com/PEP/Reports/Phase2009/RP2I/. Retrieved 2009-11-19.
- ↑ "Ethylene Glycols (Singapore) Pte Ltd, Singapore". Manufacturing locations. Shell Chemicals. http://www.shell.com/home/content/chemicals/products_services/our_products/ethylene_oxide_glycols/ethylene_oxide/manufacturing_locations/ethylene_oxide_singapore/ethylene_oxide_singapore.html. Retrieved 2009-10-12.
- ↑ "Overview". Mitsubishi Chemical Corporation. http://www.m-kagaku.co.jp/english/corporate/index.html. Retrieved 2009-10-12.
- ↑ "Shell Chemical LP – Geismar, United States of America". Manufacturing locations. Shell Chemicals. http://www.shell.com/home/content/chemicals/products_services/our_products/ethylene_oxide_glycols/ethylene_oxide/manufacturing_locations/geismar/ethylene_oxide_geismar.html. Retrieved 2009-10-12.
- ↑ "Shell Nederland Chemie BV – Moerdijk, Netherlands". Manufacturing locations. Shell Chemicals. http://www.shell.com/home/content/chemicals/products_services/our_products/ethylene_oxide_glycols/ethylene_oxide/manufacturing_locations/moerdijk/ethylene_oxide_moerdijk.html. Retrieved 2009-10-12.
- ↑ "Plants/Facilities and Capacity". CNOOC and Shell Petrochemicals Company Limited. http://www.cnoocshell.com/home/topic.aspx?topic=38. Retrieved 2009-10-12.
- ↑ "Segment Chemicals – Products". BASF. http://www.report.basf.com/2008/en/subjects/products/chemicals.html. Retrieved 2009-10-12.
- ↑ "Ethylene Oxide (EO)". Ineos Oxide. http://www.ineosoxide.com/21-Ethylene_Oxide__EO_.htm. Retrieved 2009-10-12.
- ↑ "Ethylene oxide product overview". Ethylene oxide. Shell Chemicals. http://www.shell.com/home/content/chemicals/products_services/our_products/ethylene_oxide_glycols/ethylene_oxide/product_overview/ethylene_oxide_overview.html. Retrieved 2009-10-08.
- ↑ "Ethylene Oxide (EO) Uses and Market Data". Chemical Intelligence. Chemical Industry News & Intelligence (ICIS.com). http://www.icis.com/v2/chemicals/9075772/ethylene-oxide/uses.html. Retrieved 2009-10-08.
- ↑ 78.0 78.1 IL Knunyants, ed (1988). "Ethylene". Chemical Encyclopedia. 5. "Soviet encyclopedia". pp. 984–985.
- ↑ Uri Zoller, Paul Sosis, ed (2008). Handbook of Detergents, Part F: Production. CRC Press. pp. 518–521. ISBN 9780824703493.
- ↑ Syed Naqvi (September 2009). 2009/RP2I/ "Process Economics Program Report 2I". PEP Peport. SRI Consulting. http://www.sriconsulting.com/PEP/Reports/Phase2009/RP2I/. Retrieved 2009-10-20.
- ↑ OMEGA delivers for ethylene glycol makers, Shell
- ↑ John J. McKetta, William A. Cunningham, ed (1984). Encyclopedia of chemical processing and design. 20. New York: Marcel Dekker, Inc. pp. 259–260. ISBN 0824724704.
- ↑ "Technology of ethanolamine". Technology. Himtek Engineering. http://www.himtek.ru/cgi-bin/index.cgi?IdS=18&IdP=9&Lang=0. Retrieved 2009-10-22.
- ↑ Chekalin MA, Passet BV, Ioffe BA (1980). The technology of organic dyes and intermediate products: A manual for technical (2 ed.). Khimiya. p. 185.
- ↑ R. J. Farn, ed (2006). Chemistry and technology of surfactants. Blackwell Publishing. p. 133. ISBN 1405126965.
- ↑ "Alkoxylation". BUSS LOOP Reactor. Buss ChemTech AG. http://www.buss-ct.com/e/reaction_technology/alkoxylation.php?navid=36. Retrieved 2009-10-21.
- ↑ "The Sohio Acrylonitrile Process". National Historic Chemical Landmarks. http://acswebcontent.acs.org/landmarks/landmarks/soh/soh_process.html. Retrieved 2009-10-22.
- ↑ "13.1.3.5. Oxidative ammonolysis of hydrocarbons". ChemAnalitica.com. 1 April 2009. http://chemanalytica.com/book/novyy_spravochnik_khimika_i_tekhnologa/06_syre_i_produkty_promyshlennosti_organicheskikh_i_neorganicheskikh_veshchestv_chast_II/5015. Retrieved 2009-10-22.
- ↑ Andreas F., Grabe K. (1969). Propylenchemie. Akademie-Verlag. pp. 117–118.
- ↑ 90.0 90.1 "Ethylene oxide". Chemical Backgrounders Index. The Environment Writer. http://www.environmentwriter.org/resources/backissues/chemicals/ethylene_oxide.htm. Retrieved 2009-09-29.
- ↑ "United States Patent 4132170. Fuel-air type bomb". http://www.freepatentsonline.com/4132170.pdf. Retrieved 2009-10-22.
- ↑ 92.0 92.1 PV Zimakova and Mr. O. Dymenta, ed (1967). "Chapter IV Methods of analysis of ethylene oxide". Ethylene oxide. Khimiya. pp. 128–140.
- ↑ "Ethylene oxide". ICSC/International Chemical Safety Cards. Institute of Industrial Safety, Labour Protection and Social Partnership. http://www.safework.ru/ilo/ICSC/cards/view/?0155. Retrieved 2009-09-21.
- ↑ "Ethylene Oxide". Health and Safety Guide. International Programme on Chemical Safety (IPCS) INCHEM. 1988. http://www.inchem.org/documents/hsg/hsg/hsg016.htm. Retrieved 2009-09-23.
- ↑ "Informational Bulletin NFPA-04N 2009". Department of Emergency Services, County of Sonoma. January 10, 2009. http://www.sonoma-county.org/des/pdf/fire/bulletins/info_bulletin_nfpa_marking2009_04n.pdf. Retrieved 2009-10-23.
- ↑ "Ethylene Oxide Safety Literature". Shell Chemicals. http://www-static.shell.com/static/chemicals/downloads/products_services/ethylene_oxide_safety_literature.pdf. Retrieved 2009-10-23.
- ↑ 97.0 97.1 "Ethylene oxide". Preserving agents. preservatives in food industry. http://www.konservanti.com/veshestva32.html. Retrieved 2009-09-25.
- ↑ Conviser S.. "The Future of Ethylene Oxide Sterilization". ICT Magazine. http://www.infectioncontroltoday.com/articles/061feat4.html. Retrieved 2009-10-23.
- ↑ 99.0 99.1 99.2 "Harmful substances. Section 4. Heterocyclic compounds. Triplex heterocyclic compounds". ChemAnalitica.com. 1 April 2009. http://chemanalytica.com/book/novyy_spravochnik_khimika_i_tekhnologa/11_radioaktivnye_veshchestva_vrednye_veshchestva_gigienicheskie_normativy/5177. Retrieved 2009-09-21.
- ↑ Collins J. L.. "Epoxy compounds". Encyclopedia of the ILO. Institute of Industrial Safety, Labour Protection and Social Partnership. http://base.safework.ru/iloenc?hdoc&nd=857300040&nh=0. Retrieved 2009-09-25.
- ↑ IARC Vol 60
- ↑ Steenland K, Whelan E, Deddens J, Stayner L, Ward E (2003). "Ethylene oxide and breast cancer incidence in a cohort study of 7576 women (United States)". Cancer Causes Control 14 (6): 531–9. doi:10.1023/A:1024891529592. PMID 12948284.
- ↑ Steenland K, Stayner L, Deddens J (2004). "Mortality analyses in a cohort of 18 235 ethylene oxide exposed workers: follow up extended from 1987 to 1998". Occup Environ Med 61 (1): 2–7. PMID 14691266.
- ↑ Carson P.A., Mumford C.J. (1994). Hazardous Chemicals Handbooks. Oxford: Butterworth-Heinemann Ltd. p. 85. ISBN 0750602783.
- ↑ Documentation for Immediately Dangerous to Life or Health Concentrations (IDLH): NIOSH Chemical Listing and Documentation of Revised IDLH Values (as of 3/1/95)
- ↑ Chemicals Regulation Directorate. "Banned and Non-Authorised Pesticides in the United Kingdom". http://www.pesticides.gov.uk/approvals.asp?id=55. Retrieved 1 December 2009.
- ↑ "Safety data for ethylene oxide". The Physical and Theoretical Chemistry Laboratory Oxford University. http://msds.chem.ox.ac.uk/ET/ethylene_oxide.html. Retrieved 2009-10-22.
External links
- EOSA Promoting the safe use of Ethylene Oxide for Sterilization
- WebBook page for C2H4O
- National Pollutant Inventory – Ethylene oxide fact sheet
- Ethylene Oxide User's Guide
- Ethylene Oxide MSDS (Material Safety Data Sheet).
- National Institute for Occupational Safety and Health – Ethylene Oxide Topic Page
- EOSA memo about Ethylene Oxide (EtO) facts

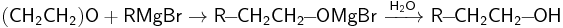

![\mathsf{(CH_2CH_2)O+HNO_3}\rightarrow\mathsf{HO\!\!-\!\!CH_2CH_2\!\!-\!\!ONO_2\ \xrightarrow[-H_2O]{+\ HNO_3}\ O_2NO\!\!-\!\!CH_2CH_2\!\!-\!\!ONO_2}](/2010-wikipedia_en_wp1-0.8_orig_2010-12/I/76dcdaa66ac039285e4924568b66f5a2.png)

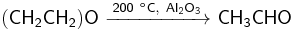
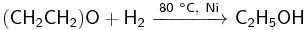
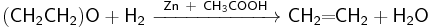
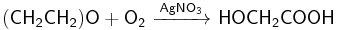




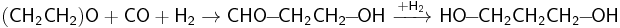
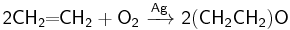
![\mathsf{(CH_2CH_2)O+CO_2}\rightarrow\mathsf{(O\!\!-\!\!CH_2CH_2\!\!-\!\!O)C\!\!=\!\!O\ \xrightarrow[-CO_2]{+H_2O}\ HOCH_2CH_2OH}](/2010-wikipedia_en_wp1-0.8_orig_2010-12/I/3489c5c452a66d58274adb6c04db8902.png)
![\mathsf{(CH_2CH_2)O+HCN}\rightarrow\mathsf{HOCH_2CH_2CN\ \xrightarrow[-H_2O]\ CH_2\!\!=\!\!CH\!\!-\!\!CN }](/2010-wikipedia_en_wp1-0.8_orig_2010-12/I/0da97cdf4b9f0a160182f0f5f3e8fae7.png)